View Procedure
| Procedure Name | Permit requirement for exportation of Virus, vaccine, Serum or an Analogous Product for treatment of Animal Diseases |
|---|
| Description |
|
Category
|
Permit requirement for exportation of Virus, vaccine, Serum or an Analogous product for treatment of Animal Diseases
|
|
Responsible Agency
|
Ministry of Livestock and Fisheries
Address: The Permanent Secretary
Ministry of Fisheries and Livestock.
Mulungushi House
P O Box 50060
Lusaka
Email: info@mfl.gov.zm
|
|
Legal base of the Procedure
|
The Animal Health Act, 2010
|
| Fee |
The validity period is 30 days
|
Associated Permits, Licences Etc.
|
Amount
|
|
Export Permits (Fishery and Livestock related)
|
ZMW 52.50
|
|
Required Documents
|
No.
|
Type of information
|
Note
|
|
1
|
Application Form
|
|
| 2 |
Proof that the importer is licensed by law to handle, use or sell the veterinary medicant or biological.
|
|
| 3 |
Commercial Invoice (or letter of sale) |
|
| 4 |
Good Manufacturing Practice (GMP) certificate |
|
| 5 |
Certification for the establishment to manufacture or produce the livestock product or by-product
|
|
| 6 |
Commercial Invoice in respect of the livestock product or by-product to be imported from the same establishment. |
|
Process Steps
|
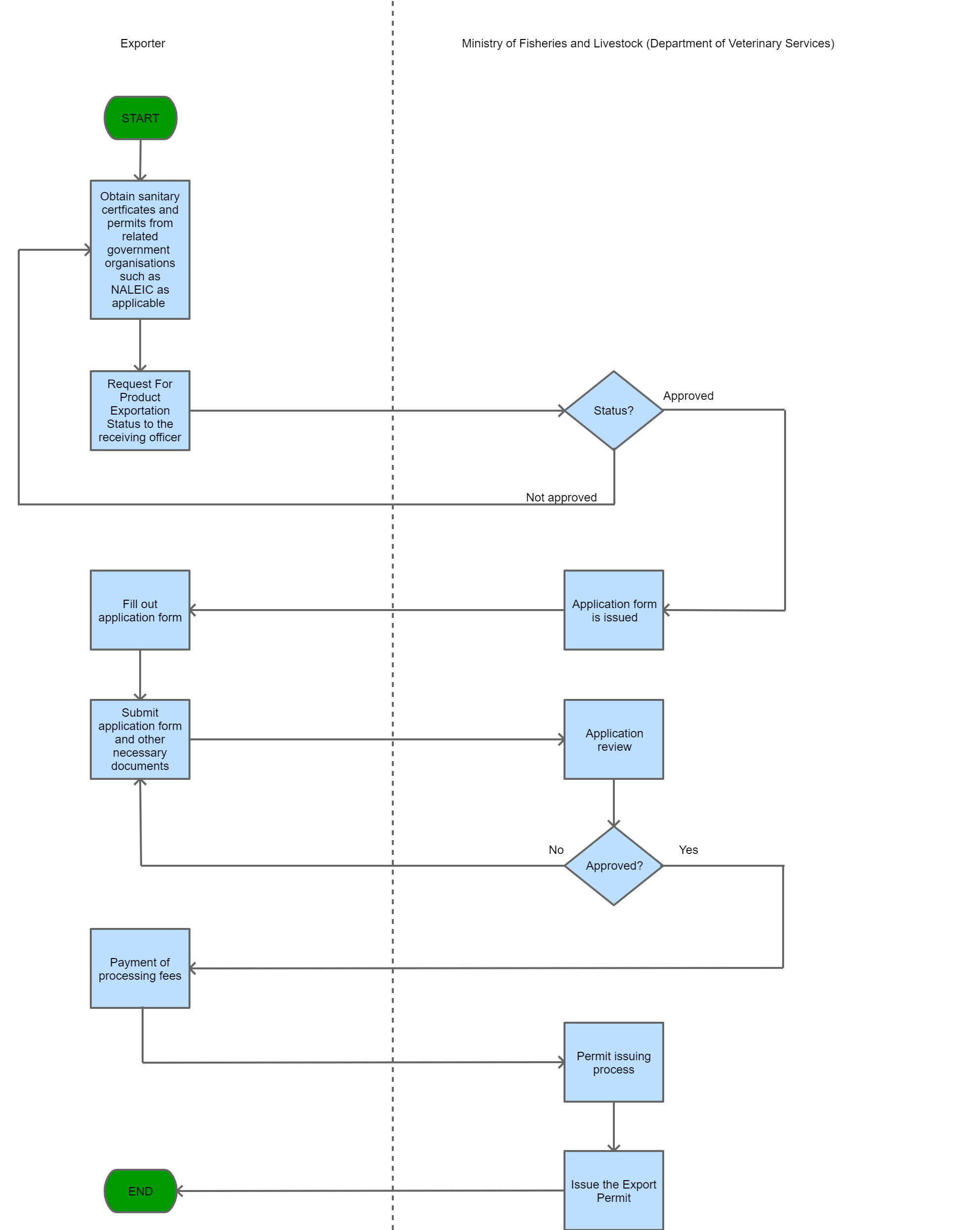
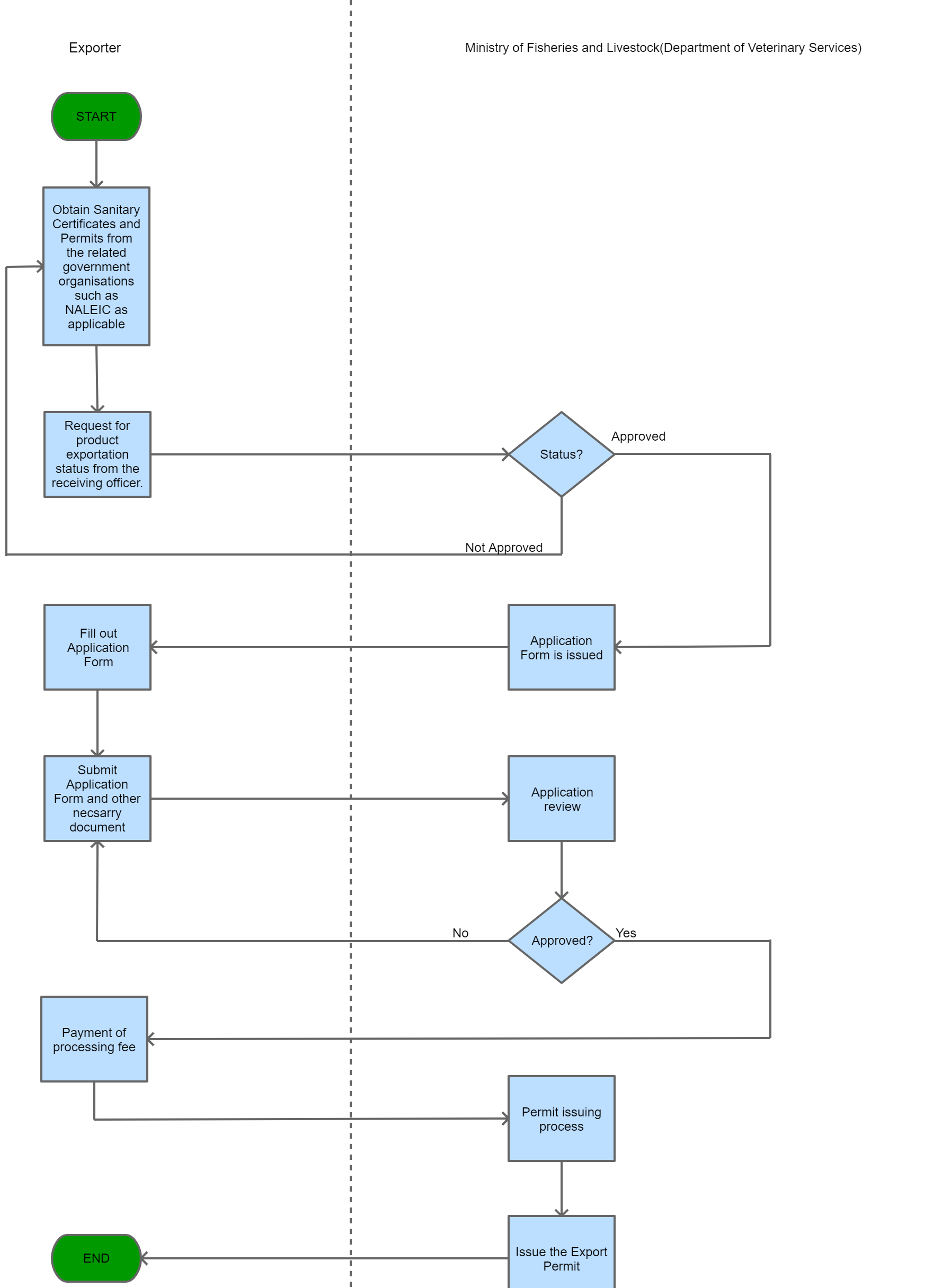
Step 1
|
The Trader makes a request for product exportation status from the receiving officer. Once this is approved, the receiving officer then issues an application form.
|
|
|
Step 2
|
The Trader completes and submits an application form along with the relevant supporting documents.
|
|
|
Step 3
|
The approving section processes the application and approves it.
|
|
|
Step 4
|
The applicant pays the processing fee and is issued with a receipt.
|
|
|
Step 5
|
The issuing section processes the application, and issues the Export Permit in writing from the Director.
|
|
| Step 6 |
A copy of the permit and related information are kept for record keeping.
|
|
| Step 7 |
the applicant may proceed with the next steps to export the goods. |
|
|---|
| Category | Export |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
| Permit requirement for exportation of Virus, vaccine, Serum or an Analogous product for treatment of Animal Diseases | Permit Requirement | | In order to export out of Zambia a virus, vaccine, serum or an analogous product used for the purpose of diagnosis or treatment of any animal disease a permit in writing issued by the Ministry of Fisheries and Livestock is required. | No virus, vaccine, serum or an analogous product used for the purpose of diagnosis or treatment of any animal disease can be exported out of Zambia without a permit in writing issued by the Ministry of Fisheries and Livestock. | The Animal Health Act, 2010
| 09-09-9999 | Good |
1288





