View Procedure
| Procedure Name | Genetically Modified Organisms (GMO's) Export Permit |
|---|
| Description |
|
ategory
|
Permit requirement for exporters
|
|
Responsible Agency
|
National Biosafety Authority
Address: Plot 100, Off Lake Road, Ibex Hill Gardens
P.O. Box 51119
Ibex Hill, Lusaka
Phone : +260 211 220 429
|
|
Legal base of the Procedure
|
The Biosafety Act, 2007
|
| Fee |
Application for a permit for the exportation of GMOs (Processing fee per Event) ZMW 5 040 |
Required Documents
|
No.
|
Type of information
|
Note
|
|
1
|
Application Letter
|
Documents necessary for the application |
| 2 |
Institutional Biosafety Committee (IBC) form |
| 3 |
Institutional Biosafety Committee (IBC) proof of registration |
| 4 |
Proof of payment of processing fee |
Process Steps
|
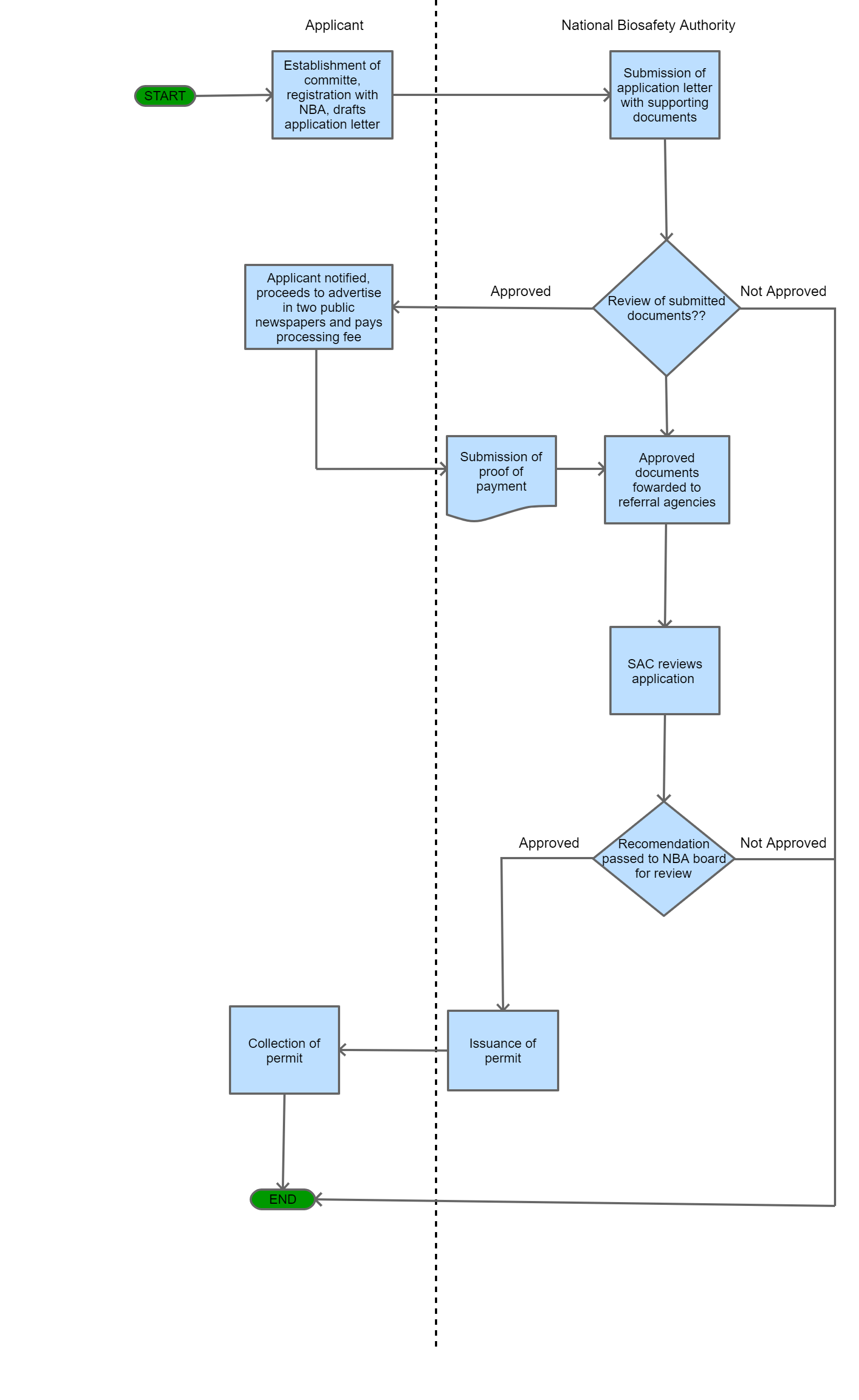
Step 1
|
An application letter should be addressed to the Registrar National Biosafety Authority (NBA) for the certification of products.
|
|
Step 2
|
The applicant submits an application letter with supporting documents.
|
|
Step 3
|
NBA Secretariat checks for completeness and accepts the application.
|
|
Step 4
|
The applicant is notified about their application status, proceeds to pay the processing fee and the authority forwards copies to appropriate referral agencies for comments
|
|
Step 5
|
The applicant proceeds to advertise in two public newspapers inviting the public to submit comments to the Authority within 30 days.
|
| Step 6 |
Scientific Advisory Committee (SAC) Reviews the application and takes into account public and referral agency comments in its final recommendations to the Board. |
| Step 7 |
The approving section (National Biosafety Authority board) then conducts a review of the recommendations by SAC and makes a final decision. |
| Step 8 |
In case of missing information or an incomplete application, NBA requests for additional or missing information from the applicant and In case of rejection, the NBA gives the applicant a notice of rejection. |
| Step 9 |
In case of the application being compliant to the Biosafety Act, the NBA issues the applicant with a permit. |
| Step 10 |
The applicant collects permit. |
|
|---|
| Category | Export |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
| Export Permit requirement for Genetically Modified Organisms | Permit Requirement | | Any person who intends to export a genetically modified organism or a product of a genetically modified organism shall provide to the National Biosafety Authority a written advance informed agreement or approval of the competent authority of the importing country and a valid export permit from the authority. | The permit to export applies to all genetically modified organisms. | The Biosafety Act, 2007 | 09-09-9999 | Good |
1248




