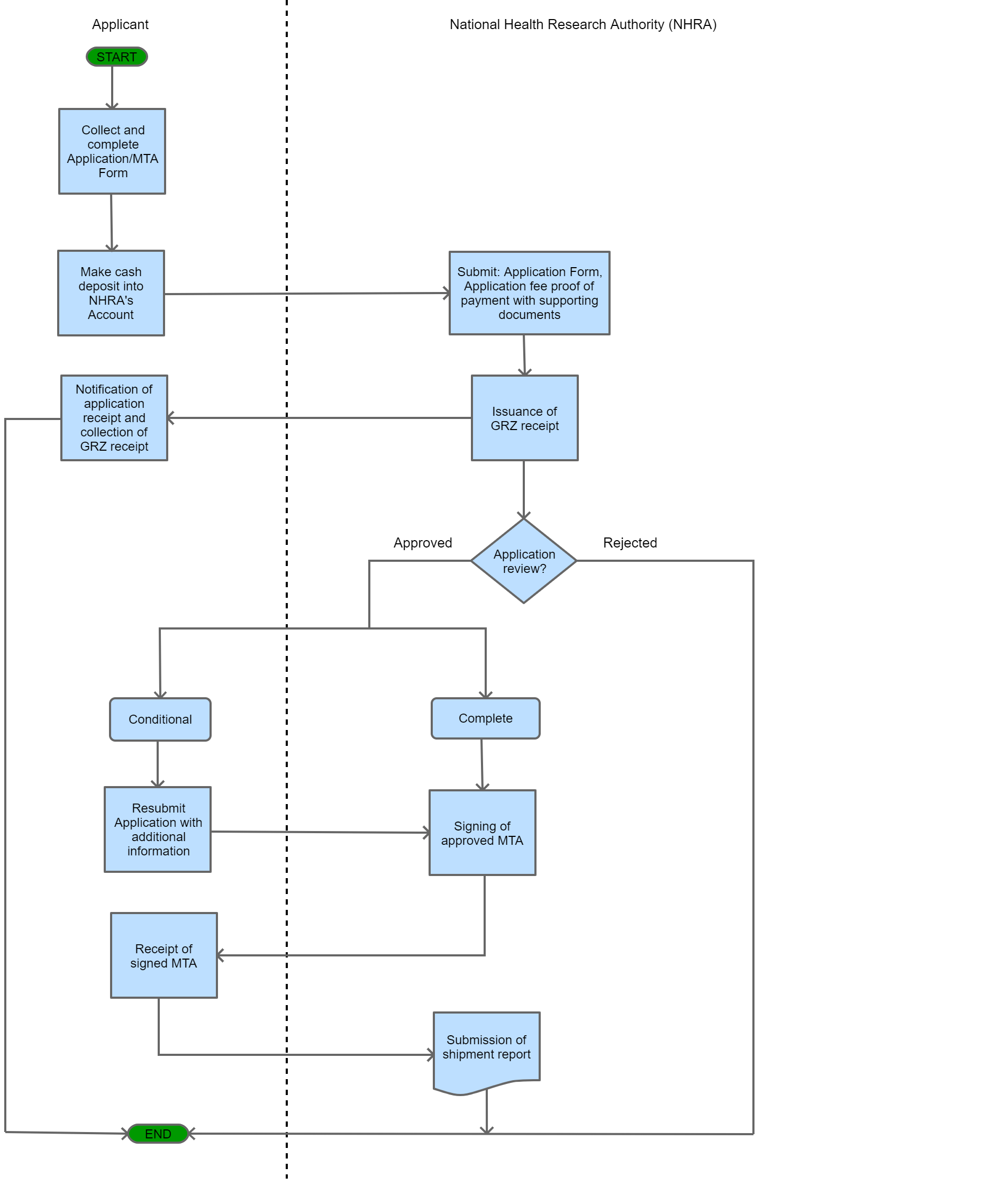
View Procedure
| Procedure Name | Import Permit for Biological Materials | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description |
Required Documents
Note: *For sequential shipments, a researcher should provide a shipment report for each shipment made. Process Steps
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Category | Import |
| Title | Description | Created Date | Updated Date | Issued By |  |
|---|---|---|---|---|---|
| Material Transfer Agreement Form-Import | Material Transfer Agreement Form for import of biological materials | 07-02-2020 | 07-02-2020 |
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
|---|---|---|---|---|---|---|---|
| Import Permit Requirement for Cultures or Pathogenic Micro-Organisms | Permit Requirement | In order to import into Zambia any culture or preparation of any pathogenic micro-organism or other material capable of causing disease in man, a person must be in possession of written permission from the Director of Medical Services therefor. | No cultures or pathogenic micro-organisms can be imported into Zambia without lawful authority. | The Public Health Act, 1930 | 09-09-9999 | Good | |
| Import Permit requirement for Biological Materials | Permit Requirement | No person shall import biological materials without the prior written approval of the National Health Research Authority. | A permit is required to import biological materials. | The National Health Research Act, 2013 | 09-09-9999 | Good |